Expanded Access Policy
Addressing the unmet medical needs of patients faced with a serious or life-threatening disease or condition is important to BOATM Biomedical. BOA’s GARNETTM product is intended to treat such serious and/or life-threatening cases of sepsis.
Compassionate Use (also known as Expanded Access)
The Compassionate Use provision in the regulations1 provides a path to access investigational products that have not yet received FDA approval or clearance. The provision permits physicians to access an investigational product to manage patients when they believe the product may benefit the diagnosis, monitoring, or treatment of the disease or condition.
Based upon the FDA Guidance Document, the following specific criteria are required for approval for Compassionate Use of an investigational product:
- The patient has a life-threatening or serious disease or condition;
- There is no comparable or satisfactory alternative therapy to diagnose, monitor, or treat the disease or condition; and
- Potential patient benefit justifies the potential risks of the investigational product.
Compassionate Use applies to products such as GARNET which is currently being studied in a clinical trial under an Investigational Device Exemption (IDE). If a physician believes that the use of GARNET would be beneficial for the treatment of an individual patient with a serious or life-threatening disease or condition outside of the IDE, the physician can apply for Compassionate Use to gain access to the GARNET product for their patient. It is important to note that GARNET has not yet received FDA approval outside the use in a clinical trial for the treatment of presumed or confirmed bloodstream infections in chronic dialysis patients. As such, the potential risks and benefits are not yet fully established. Physicians and patients should consider all possible benefits and risks when considering access to GARNET under the Compassionate Use provision of the regulation to treat a life-threatening or serious disease or condition.
In Vitro Binding of Multidrug-Resistant (MDR) Pathogens by the GARNETTM Platform Technology
Ongoing concerns exist that the overuse of antibiotic has contributed to antimicrobial resistance to many drugs that formally eradicated these pathogens (classified as “Urgent Threats” by the CDC). The World Health Organization (WHO) has declared that antimicrobial resistance is one of the top ten global public health threats. GARNET’s platform technology, FcMBL engineered human protein, has been shown to bind and remove a wide variety of pathogens during blood filtration. Several publications (2,3) reference the binding of methicillin-resistant Staphylococcus aureus (MRSA), an urgent threat, to FcMBL.
About the GARNETTM Product and Potential Relevance to COVID-19
BOA Biomedical has developed an approach for the potential treatment of patients with bloodstream infections and/or sepsis. The BOA GARNET product is a standard dialysis filter coated with a genetically engineered human protein called FcMBL. The filter will perform routine dialysis, while the addition of the FcMBL is designed to facilitate the binding of a broad spectrum of pathogens and pathogen fragments or debris called pathogen-associated molecular patterns (PAMPs). The FcMBL protein has been shown to bind to more than 100 different pathogens, including gram-positive bacteria, gram-negative bacteria, fungi, parasites, and viruses2. The coronavirus disease 2019 (COVID-19) worldwide pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). BOA Biomedical has conducted in vitro studies demonstrating the ability of the FcMBL protein (the unique binding component of GARNET) to bind the SARS-CoV-2 Spike Protein S2, which is the component of the virus that gives it access to the host cells. The proposed approach to use GARNET for the removal of pathogens from the patient’s blood involves attaching the GARNET product to a standard hemodialysis circuit. As the blood travels through the filter, viruses (such as SARS-CoV-2), bacteria, and PAMPS are potentially adsorbed and removed.
The GARNET product also proposes to remove circulating PAMPs that trigger the hyperinflammatory response, termed “cytokine release syndrome”, which may occur in patients with a viral, bacterial, or fungal infection. BOA Biomedical is currently enrolling patients in a multicenter clinical trial to demonstrate the safety of GARNET to treat patients with suspected or confirmed bloodstream infections, regardless of the underlying inciting pathogen. Complete clinical safety and/or efficacy has therefore not yet been established for GARNET.
GARNET Product Current Clinical Trial
Clinical trials are intended to evaluate the safety and efficacy of a therapy. BOA Biomedical is currently sponsoring a clinical trial assessing the GARNET product (clinicaltrials.gov identifier NCY04658017). Details of the current clinical trial are provided on the website: https://www.clinicaltrials.gov/ct2/show/NCT04658017?term=NCT04658017&draw=2&rank=1
In Vitro Clearance of COVID-19 by GARNETTM
In an in vitro study, GARNET demonstrated over 88% clearance of the SARS-CoV-2 Spike Protein S2 within 2 hours of treatment with GARNET (Figure 1). In this experimental study, a total of 250 mL of buffer sample spiked with 80 ng/mL of SARS-CoV-2 Spike Protein S2 was re-circulated in vitro through a circuit with GARNET for 2 hours at a flow rate of 300 mL/min under room temperature. SARS-CoV-2 Spike Protein 2 samples were taken at 0, 0.5, 1, and 2 hours and measured directly using BOA’s proprietary assay (referred to as AGATETM). Moreover, GARNET has the potential to clear pathogens and associated PAMPs that trigger the development of the subsequent severe inflammatory response to COVID-19, known as the cytokine release syndrome. This exuberant inflammatory response accounts for many of the adverse sequelae of SARS-CoV-2 infection.
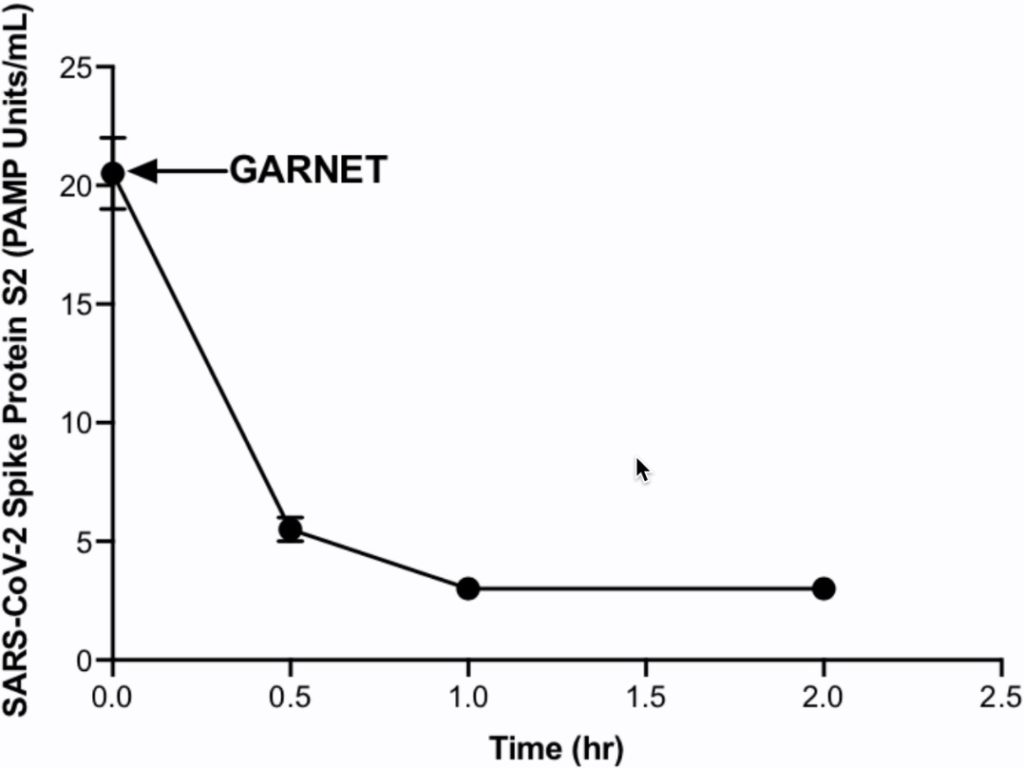
Figure 1- In vitro SARS-CoV-2 Spike Protein S2 clearance by GARNET.
In Vitro Clearance of Fungus by the GARNET™ Platform Technology
Recent reports of fungal infections accompanying COVID-19 in critically ill patients has prompted the Centers for Disease Control and Prevention (CDC) to declare the multidrug-resistant Candida auris to be a severe global threat. Unfortunately, the inability for standard laboratory culture methods to secure a timely and accurate diagnosis of this organism contribute to delayed appropriate therapeutic measures, as well as outbreaks in healthcare settings.
GARNET’s FcMBL technology has been shown to bind the mannan, a key molecule in most fungi. Furthermore, previous studies and several publications have demonstrated efficient FcMBL binding to Candida auris.2,3 These findings support the hypothesis that the GARNET filter may offer a potential to address fungal infections, including the multidrug-resistant Candida auris.
In an in vitro study, GARNET demonstrated over 83% clearance of mannan (from Saccharomyces cerevisiae fungus) within 2 hours of treatment (Figure 2). In this experimental study, a total of 500 mL of buffer sample spiked with 30 ng/mL of mannan was re-circulated in vitro through a circuit with GARNET for 2 hours at a flow rate of 300 mL/min at room temperature. Samples were collected at 0, 0.5, 1, and 2 hours and measured directly using BOA’s proprietary assay (referred to as AGATETM).
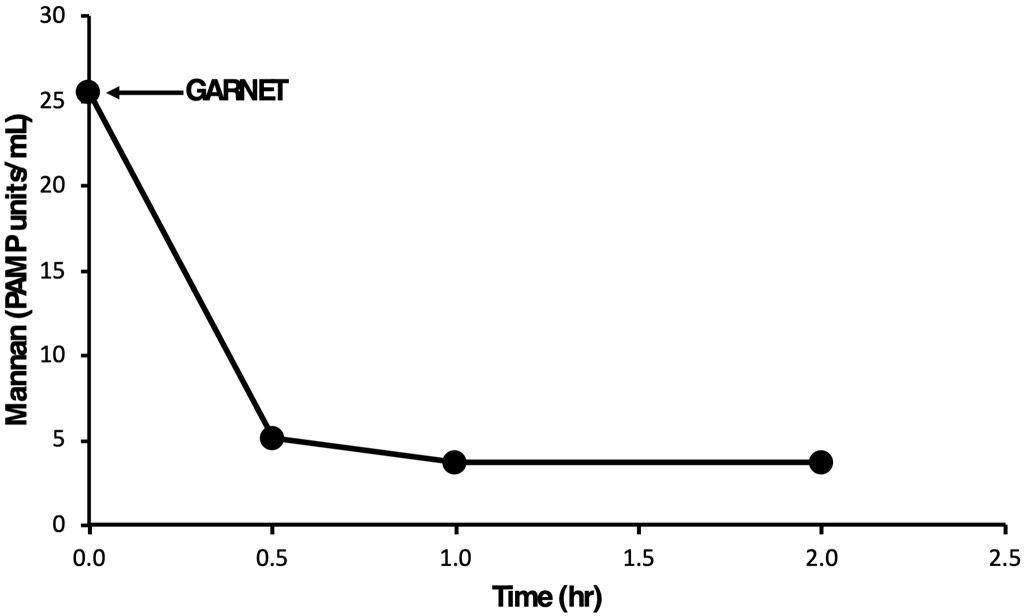
Figure 2- In vitro Mannan (from the Saccharomyces cerevisiae fungus) clearance by GARNETTM*
Moreover, GARNETTM has the potential to clear pathogens and associated PAMPs that trigger the development of the subsequent severe inflammatory response to COVID-19, known as the cytokine release syndrome. This exuberant inflammatory response accounts for many of the adverse sequelae of SARS-CoV-2 infection.
PROCESS FOR REQUESTING COMPASSIONATE USE
If a physician or patient is interested in exploring GARNETTM for the treatment of a serious or life- threatening disease or condition, the treating physician may initiate a request for “Compassionate Use” to BOA Biomedical as outlined below. The BOA Biomedical team will work collaboratively with their medical advisors and the treating physician to evaluate each reque
____
References:
- https://www.fda.gov/news-events/public-health-focus/expanded-access
- Seiler, et al., Broad-spectrum capture of clinical pathogens using engineered Fc-mannose-binding lectin enhanced by antibiotic treatment, F1000 Research Article, 8:108, January 2019.
- Cartwright, et al., A Broad-Spectrum Infection Diagnostic that Detects Pathogen-Associated Molecular Patterns (PAMPs) in Whole Blood, EBioMedicne 9, 2016, 217-227.
*Data on file at BOA Biomedical research and development. Not published.